The MTC-Q Real-Time PCR Kit is a CE-IVD molecular diagnostic assay designed for rapid detection of the Mycobacterium tuberculosis complex (MTBC) in clinical samples.
This assay supports early and accurate diagnosis of tuberculosis (TB), enabling timely treatment initiation and helping prevent delayed care, disease progression, and transmission through a standardized real-time PCR workflow.
The MTC-Q Real-Time PCR Kit is an in vitro diagnostic (IVD) assay based on real-time PCR technology for qualitative detection of MTBC-specific DNA in pulmonary and extrapulary clinical specimens.
The assay uses multiplex probe-based detection targeting two highly specific TB genomic regions:
An internal control is included to ensure sample quality and assay validity.
This dual-target design enhances diagnostic sensitivity and specificity for reliable TB detection.

Tuberculosis (TB) remains one of the world’s leading infectious diseases, caused primarily by Mycobacterium tuberculosis.
The kit is ideal for clinical molecular laboratories performing:

Targets both IS6110 and MPt64, improving diagnostic confidence across MTBC strains.
Simultaneous detection of pathogen targets plus internal control in a single reaction.
Enables identification of low bacterial load in clinical samples.
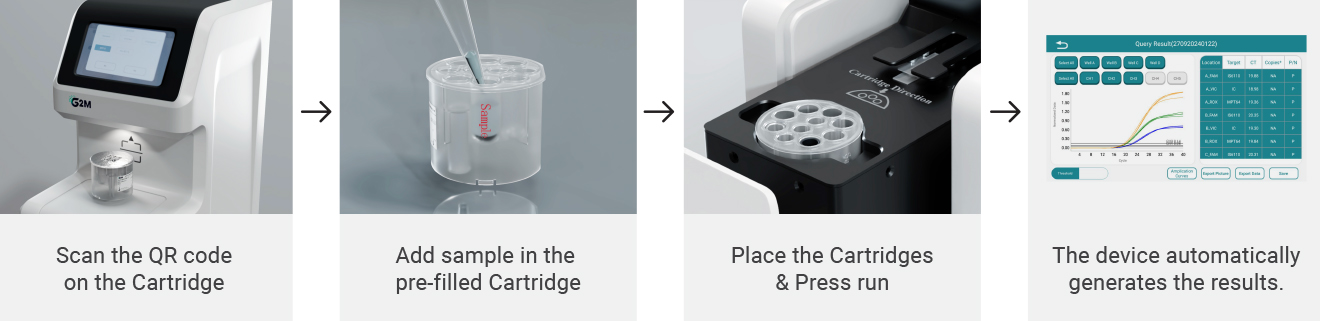
Integrated nucleic acid extraction + amplification via Genes2Me cartridge systems.
Supports faster TB diagnosis compared with traditional microbiology methods.
Validated for use with:
The assay provides clear qualitative reporting, including:
Designed for automated workflow including extraction and amplification.
| Commercial Name | Old Cat No. | New Cat No. | Pack Size |
|---|---|---|---|
| MTC-Q Real-Time PCR Kit | G2M707321R | G610014-3 | 24 Tests |
If your lab requires a reliable tuberculosis PCR test kit for MTBC detection, this assay delivers:
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.