Lung cancer, also known as bronchogenic carcinoma, originates in the lung tissue or bronchial passages and stands as one of the most formidable health challenges of our time. It is the most frequently diagnosed cancer worldwide, responsible for over 12% of all cancer cases. More alarmingly, it remains the leading cause of cancer-related deaths, claiming millions of lives each year.
Lung cancer primarily falls into two major categories: Non-Small Cell Lung Carcinoma (NSCLC) and Small Cell Lung Carcinoma (SCLC).
Recognizing the type of lung cancer is crucial, as it directly influences treatment strategies and outcomes. Early detection significantly increases the chances of survival. Next Generation Sequencing enhances the detection, and management of lung cancer by providing detailed genetic insights that inform personalized treatment approaches, improve monitoring strategies, and contribute to ongoing research efforts by allowing for the simultaneous analysis of multiple genes, providing a comprehensive view of the tumor's genetic landscape, quantify TMB, which may predict responses to immunotherapy.
The G2M FOCUS Lung is a somatic NGS assay, aimed to screen important and guideline recommended genes and fusions (like ALK, ROS1, NRGQ, RET) associated with various lung carcinomas like NSCLC (ALK, ROS1, NTRK, RET etc.) and Lung adeno-carcinomas (EGFR, MET, KRAS, BRAF etc.). The genes are selected based on the guidelines of the NCCN, CAP, ESMO, and FDA.
Consistent Mean and Median Coverage Across Key Genes
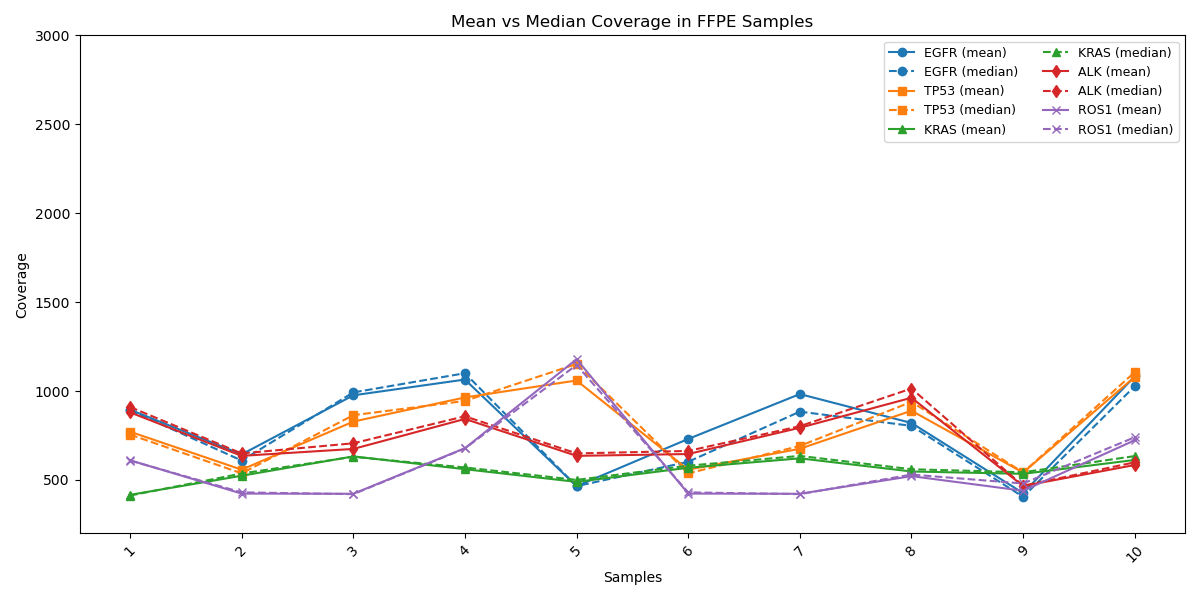
Uniform Gene Coverage Across Cancer Samples: Coverage of key cancer-associated genes (EGFR, TP53, KRAS, ALK and ROS1) shows strong alignment between mean (average depth) and median (central tendency), indicating minimal bias and consistent sequencing across target regions. This convergence reflects a robust, reliable assay suitable for routine clinical testing across diverse samples.
On-Target Alignment Across Samples
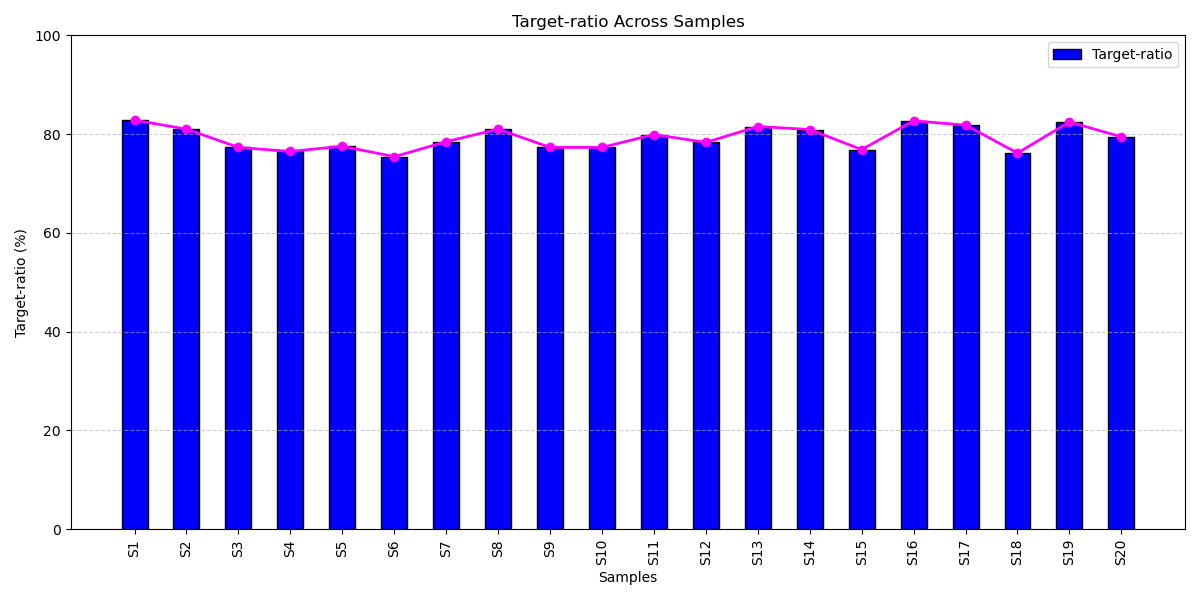
High-Efficiency Target Capture for Confident Results: All patient samples achieved over 75% on-target alignment, demonstrating the panel’s advanced design, optimized assay performance, and exceptional target enrichment, delivering consistent, high-quality sequencing that supports both clinical reliability and scalable diagnostic impact.
| Commercial Name | Old Cat No. | New Cat No. | Pack Size | Platform |
|---|---|---|---|---|
| Focus Lung-Targeted NGS Panel | G2MBR4-0979 | G2MBR4-0979 | 24 T | Illumina |
| G2MBR4-0979 | G2MBR4-0980 | 96 T | Illumina | |
| G2MBR4-0979 | G2MBR4-0981 | 96 T - EZY | Illumina – EZY | |
| Focus Lung-Targeted NGS Panel | G2MBR4-0979 | G2MBR4-0982 | 24 T | MGI |
| G2MBR4-0979 | G2MBR4-0983 | 96 T | MGI | |
| G2MBR4-0979 | G2MBR4-0984 | 96 T – EZY | MGI – EZY | |
| Focus Lung-Targeted NGS Panel | G2MBR4-0979 | G2MBR4-0988 | 24 T | Aviti |
| G2MBR4-0979 | G2MBR4-0989 | 96 T | Aviti | |
| G2MBR4-0979 | G2MBR4-0990 | 96 T – EZY | Aviti – EZY | |
| Focus Lung-Targeted NGS Panel | G2MBR4-0979 | G2MBR4-0985 | 24 T | Thermo |
| G2MBR4-0979 | G2MBR4-0986 | 96 T | Thermo | |
| G2MBR4-0979 | G2MBR4-0987 | 96 T – EZY | Thermo – EZY |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.