STI7-Q Real-Time PCR Kit (POCT)
Example report displays detection outcomes for each STI target, internal control results, and amplification plots for validation.


Fast, reliable detection of major sexually transmitted pathogens at the point of care, optimized for portable OnePCR systems and field-based sexual health screening.

The STI7-Q Real-Time PCR Kit (POCT) is an advanced in vitro diagnostic assay for the rapid and qualitative detection of seven common sexually transmitted infections (STIs), in a single test. Designed for portable OnePCR systems, it brings laboratory-grade molecular diagnostics directly to community clinics and field health programs. With its lyophilized format, the kit eliminates cold chain requirements, ensuring easy handling, transport, and stability — making it ideal for decentralized and low-resource diagnostic environments.
| Targets Covered | |
|---|---|
| Chlamydia trachomatis (C. trachomatis) | |
| Neisseria gonorrhoeae (N. gonorrhoeae) | |
| Mycoplasma genitalium (M. genitalium) | |
| Mycoplasma hominis (M. hominis) | |
| Ureaplasma urealyticum (U. urealyticum) | |
| Ureaplasma parvum (U. parvum) | |
| Trichomonas vaginalis (T. vaginalis) | |
Mobile Clinics - On-site detection and counseling
Public Health Agencies - STI prevalence monitoring
Community Programs & NGOs - Mass screening campaigns
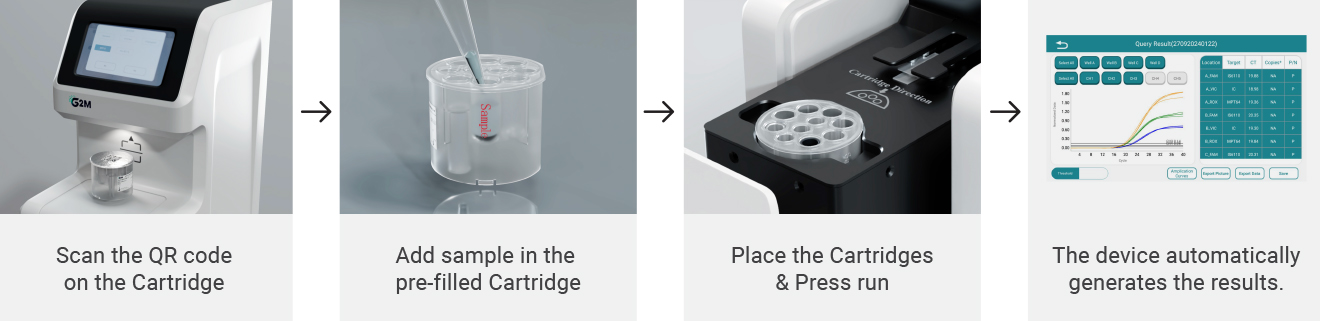
Example report displays detection outcomes for each STI target, internal control results, and amplification plots for validation.


| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| STI7-Q Real-Time PCR Kit | G2M709121R | 24T | OnePCR POC device |
| STI7-Q Real-Time PCR Kit | G2M709121R | 48T | Rapi-Q POC Device |
Accurate, portable, and clinically reliable STI detection.
CE-IVD certified and validated for decentralized, point-of-care testing environments.
Adopted by community health programs and field labs for accurate STI screening.
No refrigeration needed — easy transport and stable in all climates.
Supports rapid intervention, patient counseling, and control of STI transmission.
Download useful documents and technical information for the STI7-Q Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.