STI-Q Comprehensive Real-Time PCR Kit (POCT)
Example report shows detection outcomes for all 12 pathogens, with internal control verification and amplification plots.


Comprehensive detection of bacterial, viral, and protozoan sexually transmitted pathogens in a single multiplex test — designed for field-based, near-patient testing with portable OnePCR systems.

The STI-Q Comprehensive Real-Time PCR Kit (POCT) is a broad-spectrum diagnostic assay developed for rapid, qualitative detection of 12 sexually transmitted infection (STI) pathogens at the point of care. Covering bacteria, viruses, and protozoa, the kit uses multiplex Real-Time PCR technology to deliver accurate and cost-effective detection across a wide range of sexually transmitted agents. Its lyophilized, ready-to-use format ensures high stability and ease of deployment in resource-limited or remote health setups.
| Targets Covered | |
|---|---|
| Bacterial STIs:- C. trachomatis, N. gonorrhoeae, M. genitalium, M. hominis, U. urealyticum, U. parvum, T. pallidum | |
| Viral STIs:- HSV-1, HSV-2, HPV (selected types), HBV, HCV | |
| Protozoal STIs:- T. vaginalis | |
| Internal Control (RNaseP):- Ensures sample quality and assay reliability | |

Public Health Departments – STI mapping and prevalence studies
Mobile Health Units – On-site multiplex detection
Community Health Centers – Integrated sexual health diagnostics
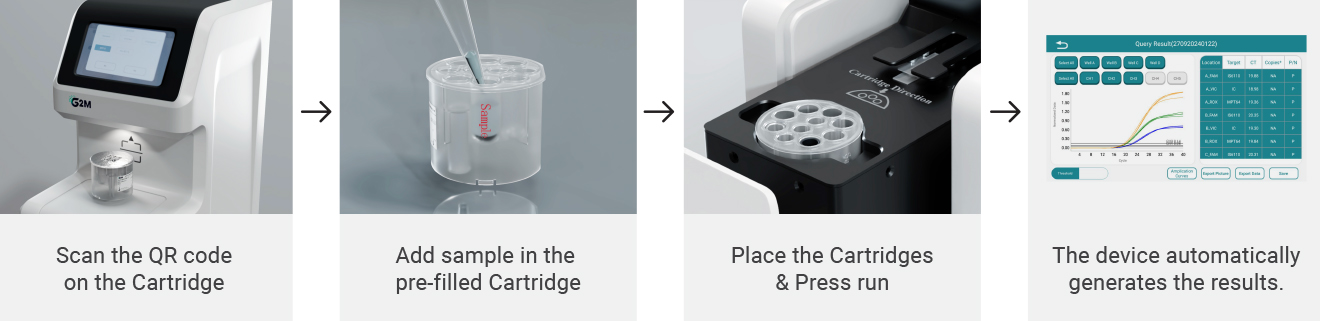
Example report shows detection outcomes for all 12 pathogens, with internal control verification and amplification plots.


| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| STI-Q Comprehensive Real-Time PCR Kit | G2M803721R | 24T | OnePCR POC device |
| STI-Q Comprehensive Real-Time PCR Kit | G2M803721R | 48T | Rapi-Q POC Device |
All-in-one, field-deployable solution for complete STI detection.
Simultaneous detection of bacterial, viral, and protozoal pathogens for full-spectrum STI diagnosis.
Proven accuracy and reproducibility across diverse sample types and field conditions.
Eliminates cold chain dependency, ensuring reliable performance in any environment.
Supports integrated sexual health programs aimed at early detection and prevention of STIs.
Download useful documents and technical information for the STI-Q Comprehensive Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.