HPV-Q Real-Time PCR Kit
Example report includes detection of HPV16, HPV18, and other high-risk genotypes, internal control validation, and result interpretation summary.


Fast, accurate detection of 14 high-risk HPV genotypes at the point of care, enabling early cervical cancer risk assessment and on-site patient management through portable OnePCR systems.
The HPV-Q Real-Time PCR Kit (POCT) is an advanced in vitro diagnostic assay designed for the rapid and qualitative detection of 14 high-risk human papillomavirus (HPV) genotypes, with differential identification of HPV16 and HPV18, the two types most commonly linked to cervical cancer. rapid, reliable, and actionable results, Optimized for portable OnePCR platforms, this point-of-care (POC) kit delivers laboratory-grade accuracy in decentralized and low-resource screening environments, supporting early detection and cervical cancer prevention initiatives.
| Targets Covered | |
|---|---|
| 14 High-Risk HPV Genotypes: HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, 68. | |
| HPV16 & HPV18: Differential detection via E6/E7 oncogenic markers. | |
| Internal Control (RNaseP): Ensures sample quality and assay reliability. | |

Community Health Camps:- On-site HPV testing and awareness programs
Clinics & Diagnostic Vans:- Rapid cervical cancer risk screening
NGO & Government Programs:- Large-scale women’s health drives
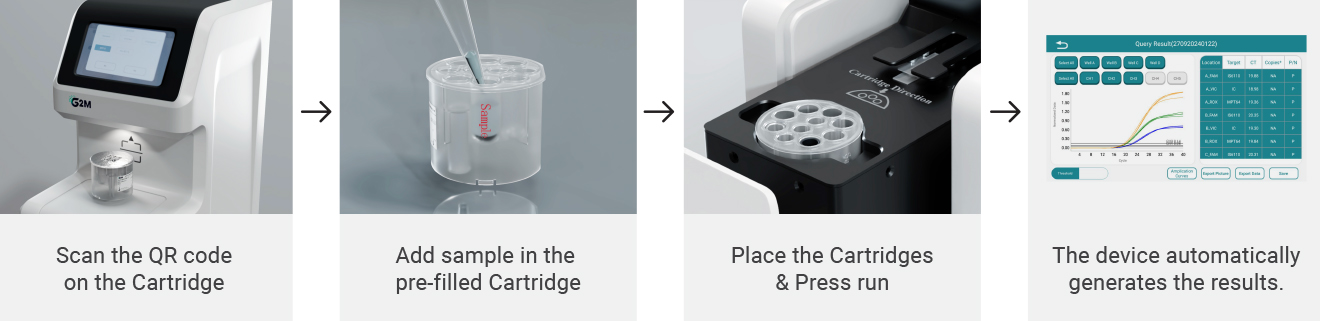
Example report includes detection of HPV16, HPV18, and other high-risk genotypes, internal control validation, and result interpretation summary.


| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| HPV-Q Real-Time PCR Kit | G2M706721R | 24T | OnePCR POC device |
| HPV-Q Real-Time PCR Kit | G2M706721R | 48T | Rapi-Q POC Device |
Reliable, portable, and accurate HPV detection technology. .
CE-IVD certified and validated on OnePCR portable platforms for POC use.
Deployed across community screening programs for early cervical cancer prevention.
Stable reagents with no cold chain required — ideal for resource-limited or remote areas.
Enables immediate HPV risk detection and clinical follow-up at the community level.
Download useful documents and technical information for the HPV-Q Real-Time PCR Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.