Early and accurate diagnosis is essential in leukemia management. The BCR-ABL Real-Time PCR Kit enables fast, qualitative detection of BCR-ABL fusion transcripts (Major, Minor & Micro breakpoints) directly from blood and bone marrow RNA. Tailored for molecular oncology testing, the kit empowers hematology centers and cancer specialists to confirm Philadelphia-chromosome–associated leukemia efficiently and guide treatment decisions with confidence.
The BCR-ABL Real-Time PCR Kit is a multiplex RT-PCR–based in-vitro diagnostic test designed for rapid, qualitative detection of BCR-ABL (Mbcr) fusion gene transcripts. The assay supports leukemia diagnosis workflows, helping clinicians classify CML and Ph-positive ALL patients quickly, even in settings without access to full cytogenetic analysis. Compatible with OnePCR® Ultra-Fast POC System and Rapi-Q® POC PCR System, the kit fits both centralized labs and point-of-care oncology units.
| Targets Detected: The panel includes | |
|---|---|
| BCR-ABL1 Major breakpoint | |
| BCR-ABL1 Minor breakpoint | |
| BCR-ABL1 Micro breakpoint | |
| Internal Control – ABL1 gene (monitors RNA integrity & assay validity) | |

Collect blood or bone marrow sample | Extract RNA & load into lyophilized PCR cartridge | Insert cartridge into OnePCR devic | Automated sample processing & multiplex RT-PCR | Results displayed as Positive / Negative + Ct values
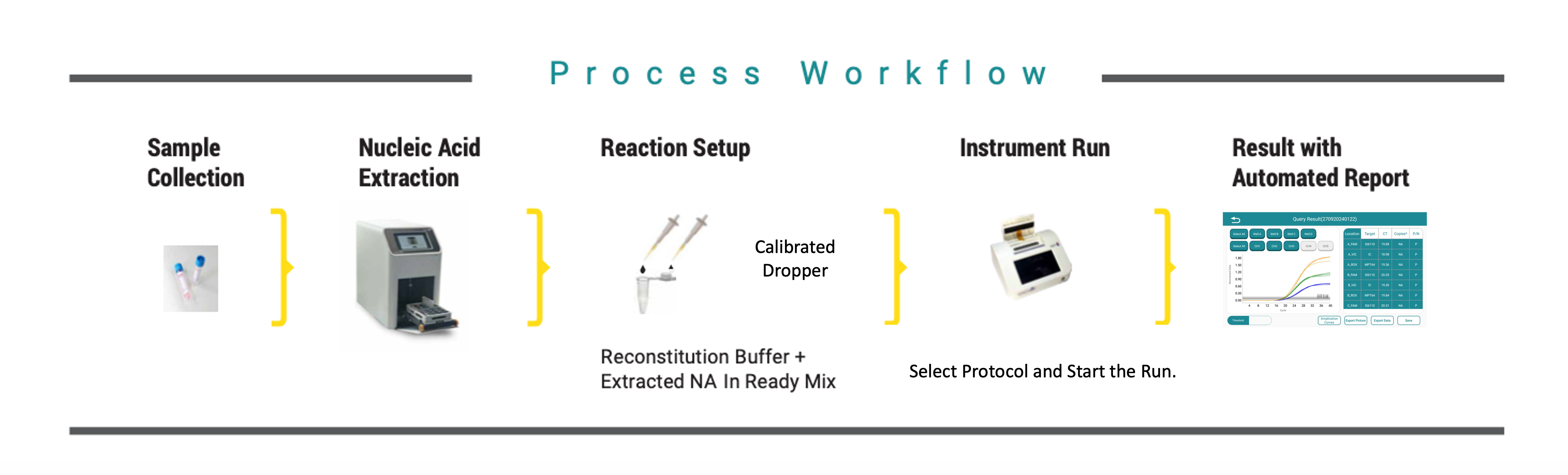
Perform RNA extraction using Rapi-X16 module | Reconstitute lyophilized ready-mix tubes | Add RNA sample & load into Rapi-Q block | Run BCR-ABL program view results in real time
| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| BCR-ABL Real-Time PCR Kit | G2M708921R - 24T | 24 Tests | OnePCR Ultra-Fast POC System |
| BCR-ABL Real-Time PCR Kit | G2M708921R - 48T | 48T | Rapi-Q Portable PCR Device |
Download useful documents and technical information for the BCR ABL RT-PCR Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
All products listed in the catalogue are the products of Genes2Me Private Limited. Apart from that, all other product names, trademarks and logos wherever used in the catalogue are the property of their respective owners.
© 2025 Genes2me. All rights reserved.