Sepsis-Q Real-Time PCR Kit
Clear Positive / Negative result for each target organism Ct values and real-time amplification curves displayed per tube Internal control readouts for run validity


Syndromic, real-time detection of sepsis-causing pathogens directly from whole blood & blood culture – optimized for OnePCR & Rapi-Q POC systems.
The Sepsis-Q Real Time PCR Kit is an in vitro diagnostic assay for the qualitative detection of sepsis-related pathogen DNA in whole blood and blood culture samples. Built on advanced Real-Time PCR technology and tightly integrated with OnePCR-Rapi-Q Ultra-Fast POC RT-PCR System and Rapi-Q POC RT-PCR System, Sepsis-Q enables: Early identification of key bacterial and fungal pathogens Support for timely, targeted antimicrobial therapy Streamlined sample-to-result workflows for emergency, ICU, NICU, and critical care settings

Collect & prepare clinical specime | Load into lyophilized PCR tube/cartridge | Insert cartridge into OnePCR device | Automated multiplex PCR amplification | Results displayed as Positive/Negative + Ct values
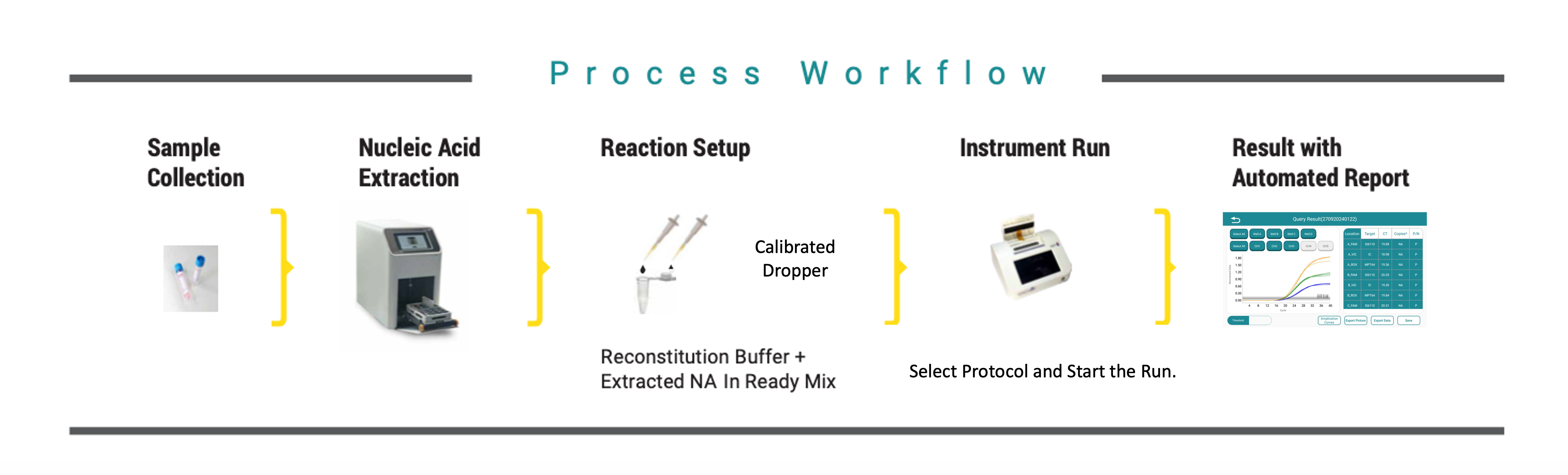
Perform DNA extraction using Rapi-X16 module | Reconstitute lyophilized reagents | Load into Rapi-Q system | Run AMR-Q program → view results in real-time
Clear Positive / Negative result for each target organism Ct values and real-time amplification curves displayed per tube Internal control readouts for run validity


| Commercial Name | Cat No. | Pack Size | Compatible Instruments |
|---|---|---|---|
| Sepsis-Q Real Time PCR Kit for OnePCR | G2M804026R-24T | 24T | OnePCR-Rapi-Q Ultra-Fast POC RT-PCR System |
| Sepsis-Q Real Time PCR Kit for Rapi-Q | G2M804026R-48T | 48T | Rapi-Q POC Rapid RT-PCR System + Rapi-X16 Extraction Module |
Comprehensive sepsis coverage – broad bacterial & fungal pathogen panel tailored to real-world ICU & NICU needs
broad bacterial & fungal pathogen panel tailored to real-world ICU & NICU needs
for flexible sampling strategies
OnePCR, Rapi-Q, Rapi-X16, and OneXtract extraction kits
High sensitivity & specificity supported by robust analytical and clinical validation
Download useful documents and technical information for the Sepsis-Q POC Kit.
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.