The BCR-ABL Quantitative Real-Time PCR Kit is a highly reliable in vitro diagnostic assay designed for the quantitative detection of BCR-ABL1 fusion transcripts in RNA extracted from blood and bone marrow samples. The BCR-ABL1 gene fusion is commonly known as the Philadelphia chromosome abnormality, which is the hallmark of Chronic Myeloid Leukemia (CML) and a subset of Acute Lymphoblastic Leukemia (ALL) cases.
The detection confirms diagnosis and plays a pivotal role in treatment planning and disease monitoring. Quantifying the expression levels of this fusion gene also enables clinicians to evaluate treatment response and minimal residual disease, ensuring timely clinical interventions for better patient outcomes.
Designed for trained laboratory professionals, the BCR-ABL Quantitative Real-Time PCR Kit uses advanced RT-PCR technology to deliver precise, sensitive, and reproducible results for leukemia management. It supports accurate diagnosis and reliable monitoring of Chronic Myeloid Leukemia (CML) and select cases of Acute Lymphoblastic Leukemia (ALL). Combining strong performance with ease of use, it enables laboratories to generate timely, actionable insights for informed clinical decisions.

Specifically designed primers and fluorescent probes detect major BCR-ABL1 transcripts (p210: e14a2 and e13a2) for accurate leukemia assessment.
Includes nine quantification standards (five for BCR-ABL1 and four for ABL1) to generate precise standard curves for reliable transcript measurement and normalization.
FAM-labeled probes target the BCR-ABL1 fusion gene, while Texas Red-labeled probes detect the ABL1 internal control, ensuring high specificity, sensitivity, and assay reliability.
Detects low transcript levels, supporting effective minimal residual disease (MRD) monitoring in leukemia patients.
Validated on leading real-time PCR platforms including QuantStudio™ 5 (Thermo Fisher), Bio-Rad CFX96, Roche LightCycler® 480, and RapiCycler 96 (Genes2Me).
Features optimized reagents and a 12-month stability profile, ensuring consistent performance and reliable outcomes across varying clinical laboratory conditions.

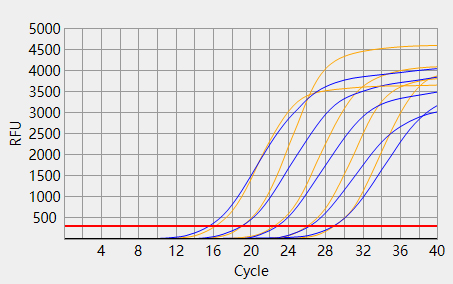
When the Ct value of the sample to be tested is outside the reference range and there is a typical S-shaped amplification curve, the test result is positive; When the Ct value of the sample to be tested is within the reference range, or the Ct value is outside the reference range but there is no typical S-shaped amplification curve, the test result is negative.
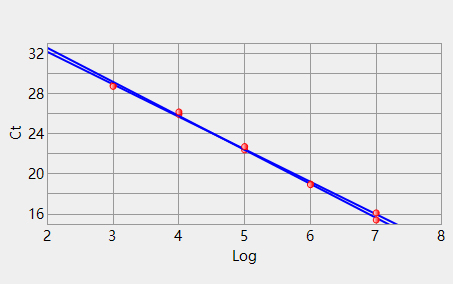
Channel 1 Slope: -3.392 Intercept: 39.372 Correlation: -0.998 Efficiency 3: 97.1430 Channel 3 Slope: -3.240 Intercept: 38.672 Correlation: -0.999 Efficiency 2: 103.52
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| BCR-ABL Quantitative Real Time PCR Kit | G2M802821 | 50 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.