The JAK2-Q Real-Time PCR Kit from Genes2Me is a high-performance molecular diagnostic solution for the rapid and accurate detection of the JAK2 V617F (1849 G>T) mutation from clinical samples. This mutation is an essential biomarker in the diagnosis of myeloproliferative neoplasms (MPNs), including polycythemia vera (PV), essential thrombocythemia (ET), and primary myelofibrosis (PMF).
With its optimized assay design, stringent quality checks, and international standard compliance, the JAK2-Q kit delivers high sensitivity, non-invasive testing, and fast turnaround times - empowering laboratories and clinicians with reliable results for early detection, treatment guidance, and disease monitoring. Combining speed, precision, and convenience, the JAK2-Q kit enhances laboratory efficiency and supports improved patient outcomes, making it a trusted choice for advanced diagnostics.
Detects the JAK2 V617F mutation at very low allelic frequencies, ensuring highly accurate and reliable results with every test.
Demonstrates a limit of detection of 0.3%, high precision (CV < 4.5%), and excellent clinical accuracy (≥96% sensitivity and ≥98% specificity).
Utilizes distinct fluorescent probes for wild-type alleles (FAM channel) and mutant alleles (HEX channel), providing robust internal control and minimizing false-negative results.
Requires only a standard blood sample, offering a convenient and patient-friendly alternative to invasive diagnostic procedures.
Delivers results within hours, enabling rapid clinical decision-making compared to conventional methods such as bone marrow biopsy or cytogenetic testing.
Optimized for major real-time PCR platforms including QuantStudio™ 5, Bio-Rad CFX96, Roche LightCycler® 480, and Genes2Me RapiCycler 96.

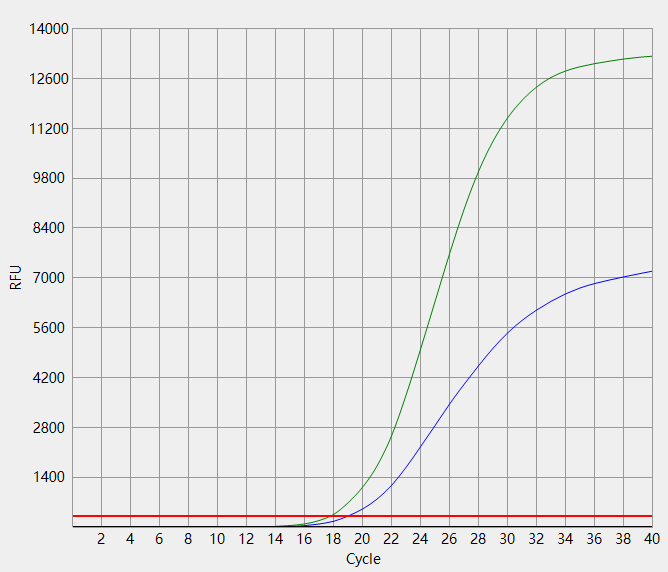
When the Ct value of the sample to be tested is outside the reference range and there is a typical S-shaped
amplification curve, the test result is positive;
When the Ct value of the sample to be tested is within the reference range, or the Ct value is outside the reference
range but there is no typical S-shaped amplification curve, the test result is negative.
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| Jak2-Q Real Time PCR Kit | G2M802621 | 50 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.