SpiNXT Blood DNA Extraction Kit is designed for rapid and reliable isolation of high-quality genomic DNA from human blood-derived samples. The kit enables consistent DNA recovery suitable for demanding molecular biology and diagnostic research workflows, ensuring reproducibility, purity, and compatibility with a wide range of downstream applications.
Kit components: Buffer LB | Buffer BW1 | Buffer BW2 | Binding Buffe | Proteinase K | Proteinase Dissolving Buffer | Buffer AE | RNaseA
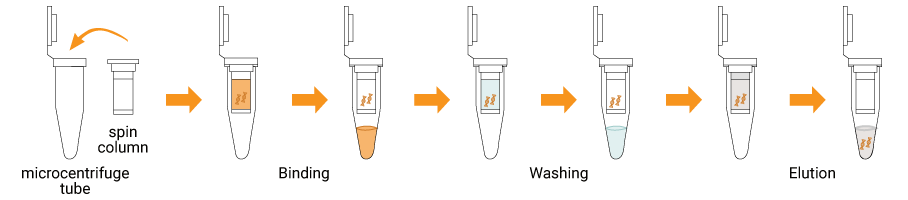
The SpiNXT Blood DNA Extraction Kit is designed for efficient isolation and purification of high-quality genomic DNA from human blood-derived samples including whole blood, plasma, serum, buffy coat, and isolated lymphocytes. The kit is compatible with blood collected in EDTA, citrate, and heparin anticoagulant tubes, ensuring flexibility across routine and archived samples. The optimized lysis chemistry enables effective disruption of blood cells and complete protein digestion, while efficiently removing common blood-derived inhibitors such as heme, anticoagulants, and enzymatic contaminants. The formulation is specifically suited for heparin-treated blood samples, overcoming PCR inhibition commonly associated with heparin and enabling reliable downstream molecular analysis. During purification, genomic DNA is selectively retained while proteins, salts, residual anticoagulants, and other impurities are removed through sequential wash steps. Purified DNA is eluted in a low-salt buffer, resulting in high-purity, intact DNA suitable for sensitive molecular biology applications.

| Format: | Mini spin column |
| Technology: | Silica membrane |
| Sample Type: | Whole Blood (200µl), Serum, plasma, Lymphocytes/Buffy coat |
| Yield: | Up to 10 µg |
| Target: | Genomic DNA |
| A260/280: | 1.6–1.9 |
| Available Pack Sizes: | 50 T, 250 T |
| Throughput Compatibility: | Manual |
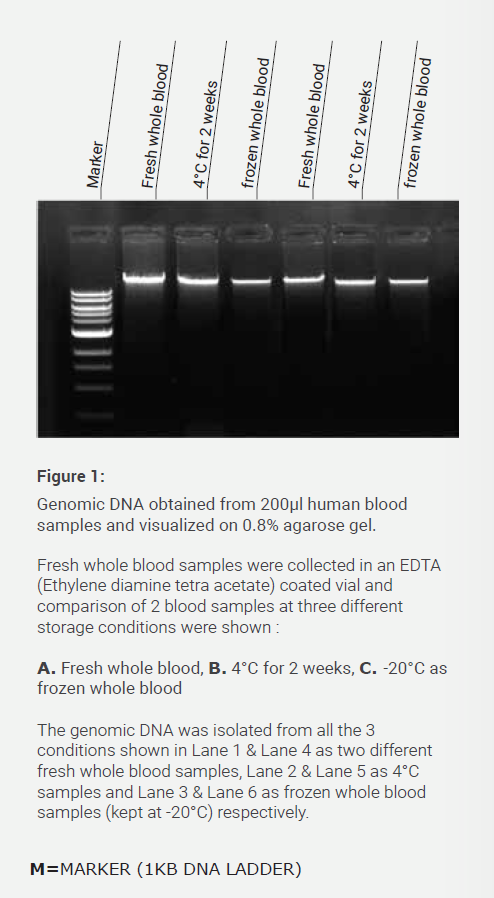
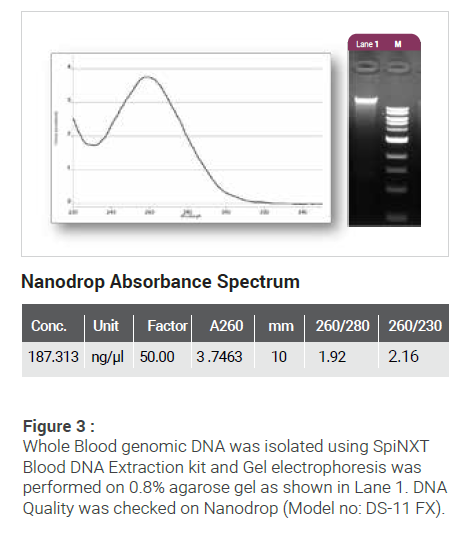
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| SpiNXT Blood DNA extraction kit | G2MBR4-0579 | 50 T |
| SpiNXT Blood DNA Extraction Kit | G2MBR4-0580 | 250 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.