The SpiNXT Bacterial DNA Extraction Kit is designed for the rapid and reliable isolation of high-quality genomic DNA from viable bacterial cells. The kit provides an efficient solution for laboratories performing microbiological and molecular biology studies and supports DNA extraction from both Gram-positive and Gram-negative bacteria. With a streamlined workflow and consistent performance, the kit enables laboratories to obtain purified bacterial DNA suitable for downstream molecular analysis.
Kit components: Buffer DLB | Buffer DLB-20 | Buffer W1 | Buffer W2 | Proteinase K | Proteinase Dissolving Buffer | Buffer EB | Lysozyme | RNaseA
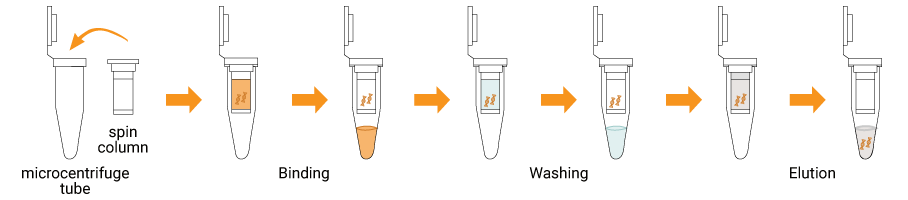
The SpiNXT Bacterial DNA Extraction Kit utilizes a silica membrane based spin column purification method for efficient extraction of bacterial genomic DNA. Bacterial cells are lysed using optimized enzymes and detergents to release DNA, while proteins and other cellular components are denatured and removed. Under appropriate binding conditions, the released DNA selectively binds to the silica membrane within the spin column. Impurities are eliminated through sequential washing steps, and purified DNA is finally eluted using a low-salt buffer or water. This method eliminates the use of hazardous chemicals such as phenol or chloroform and avoids alcohol precipitation, resulting in minimal handling and improved laboratory safety. The purified bacterial DNA is suitable for a wide range of downstream molecular biology applications and can be seamlessly integrated into routine microbiological and research workflows.
| Format: | Mini Spin Column |
| Technology: | Silica membrane |
| Sample Type: | Bacterial Culture |
| Yield: | >4µg |
| Target: | DNA |
| A260/280: | 1.6 - 1.9 |
| Available Pack Sizes: | 50 T, 250 T |
| Throughput Compatibility: | Manual |
| Commercial Name | Cat No. | Pack Size |
|---|---|---|
| SpiNXT Bacterial DNA Extraction Kit | G2MBR4-0642 | 50 T |
| SpiNXT Bacterial DNA Extraction Kit | G2MBR4-0643 | 250 T |
Since its inception in 2016, Genes2me has been constantly striving towards setting a benchmark in the diagnostics space by introducing premium quality (Made in India) diagnostic kits which are CE-IVD, ISO-13485:2016, and ISO 9001:2015 certified, assuring our clients of unparalleled quality and compliance with international standards.
© 2025 Genes2me. All rights reserved.